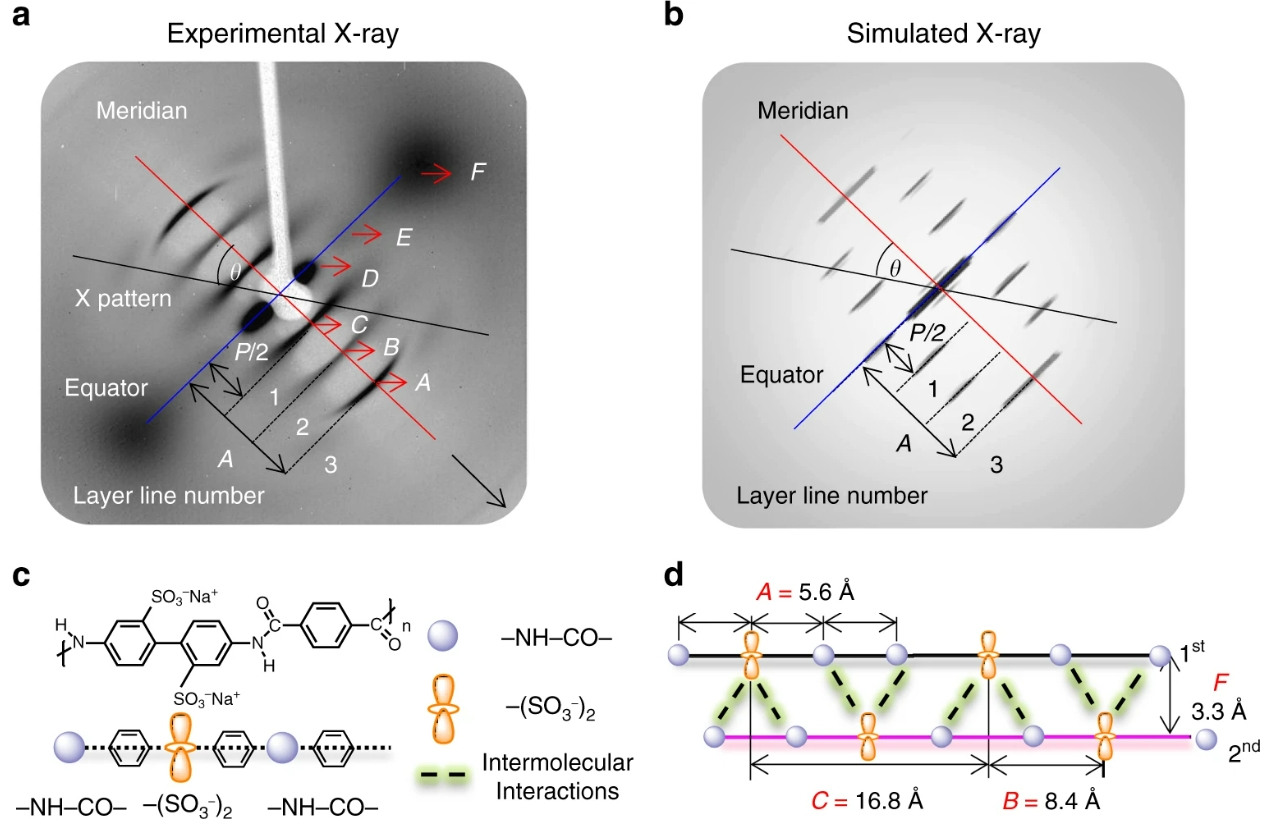
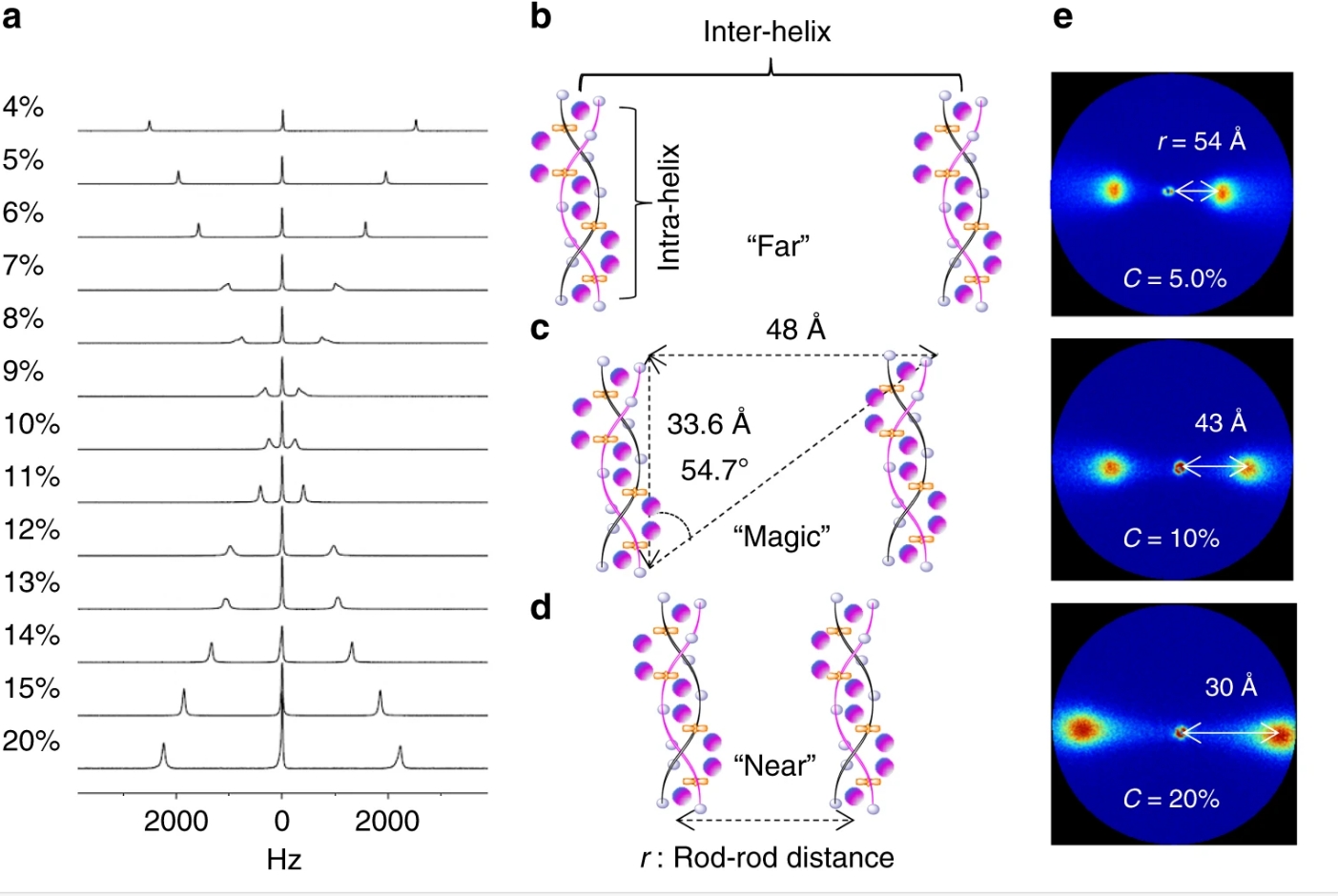
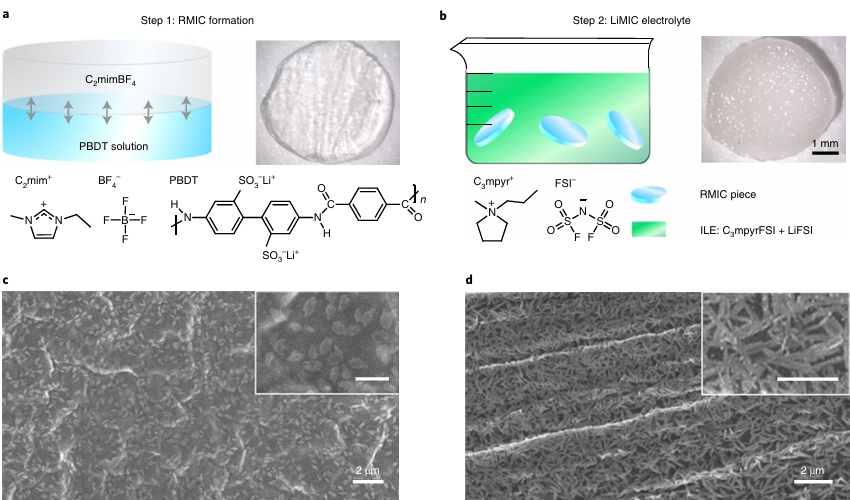
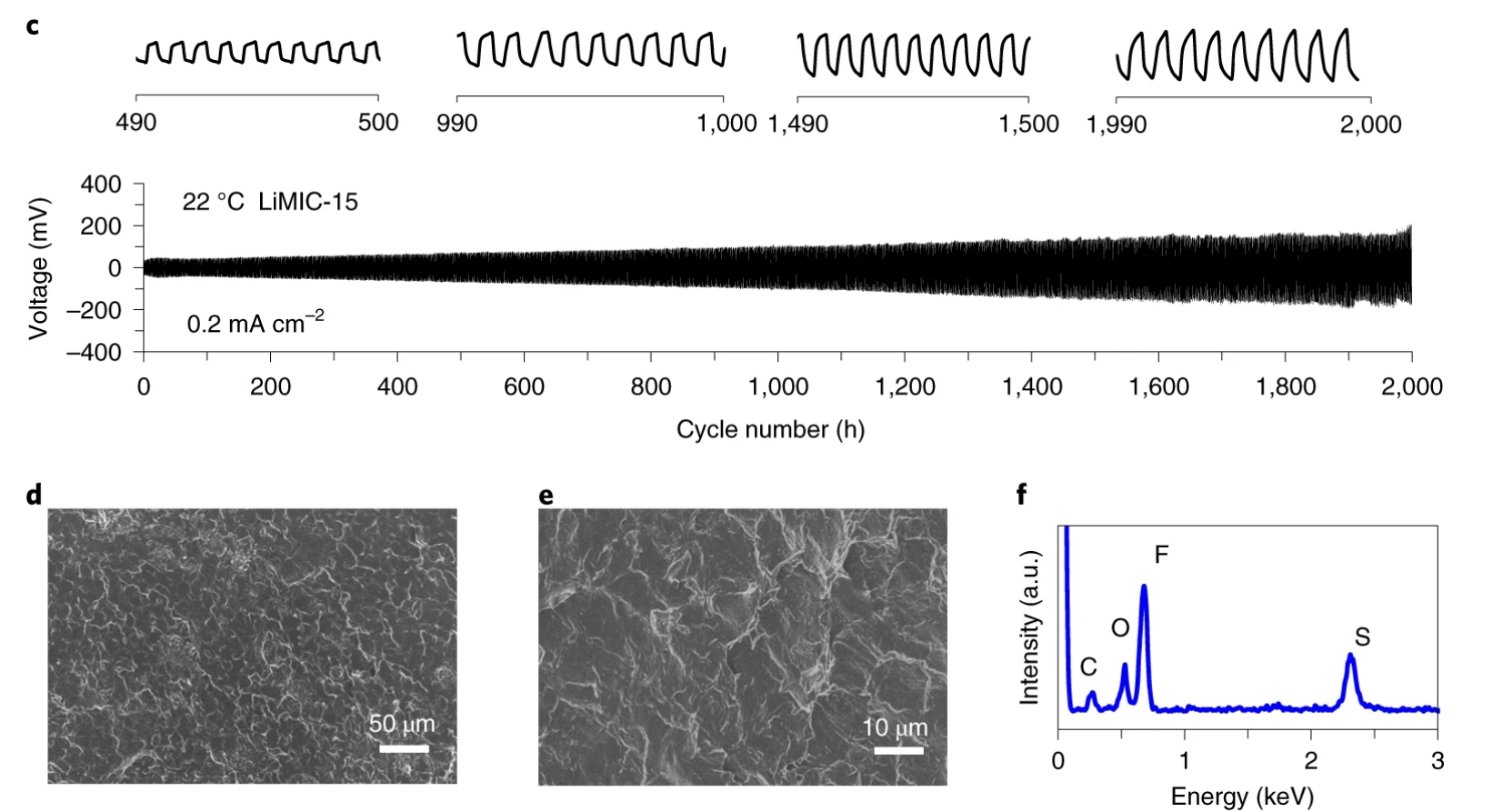
POLYMER
Polymer synthesis and self-assembly behavior and dynamics

Electrolytes
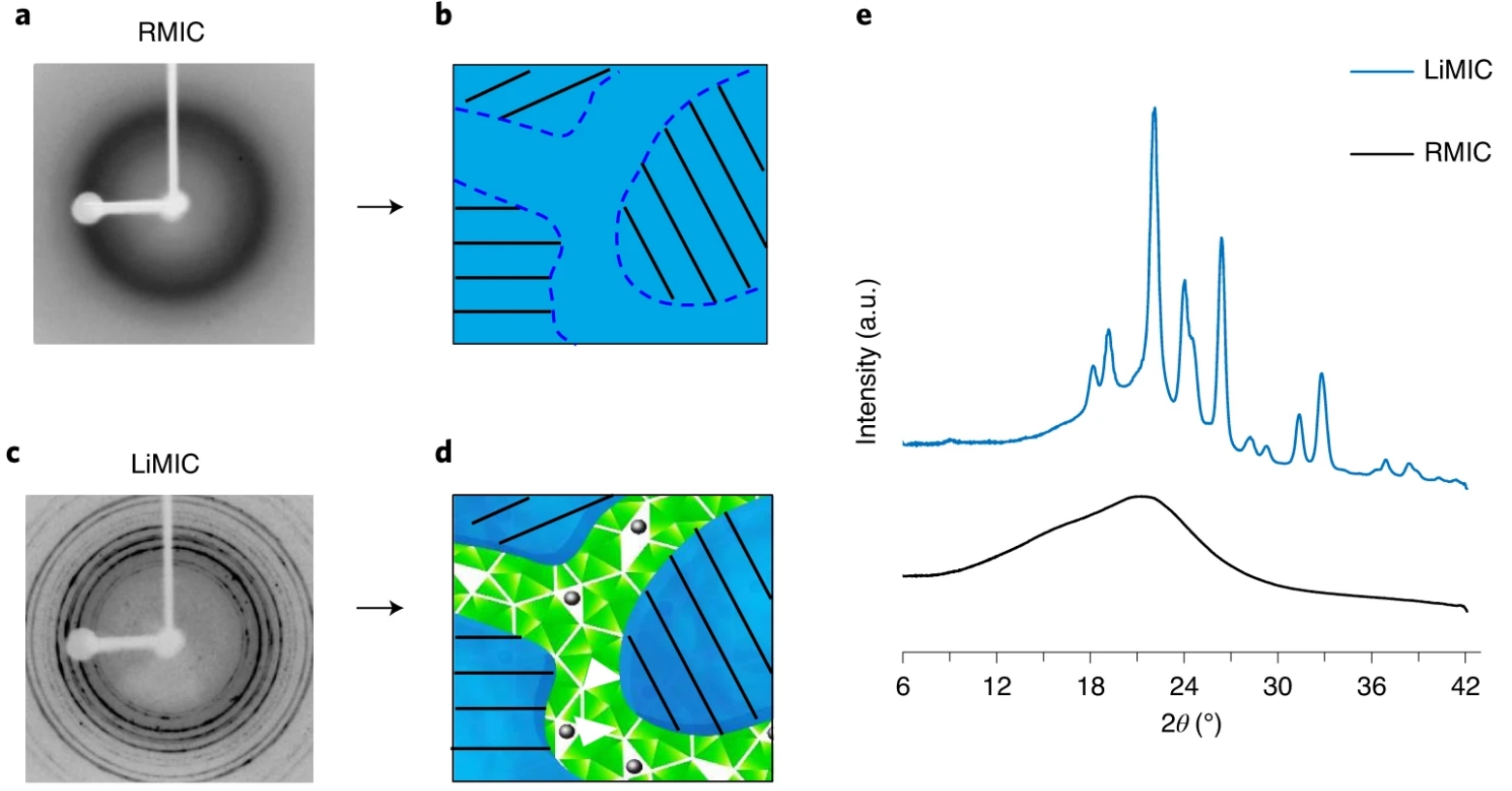
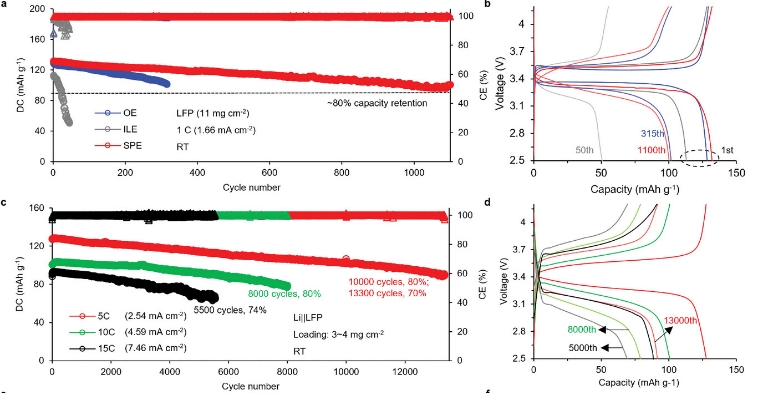
Development and characterization of solid-state polymer electrolytes

Mechanism
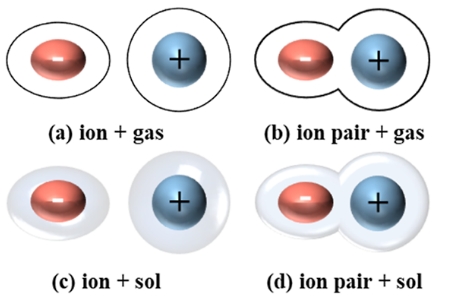
Investigation of ion transport and phase transition in solid-state electrolytes
AI/ML
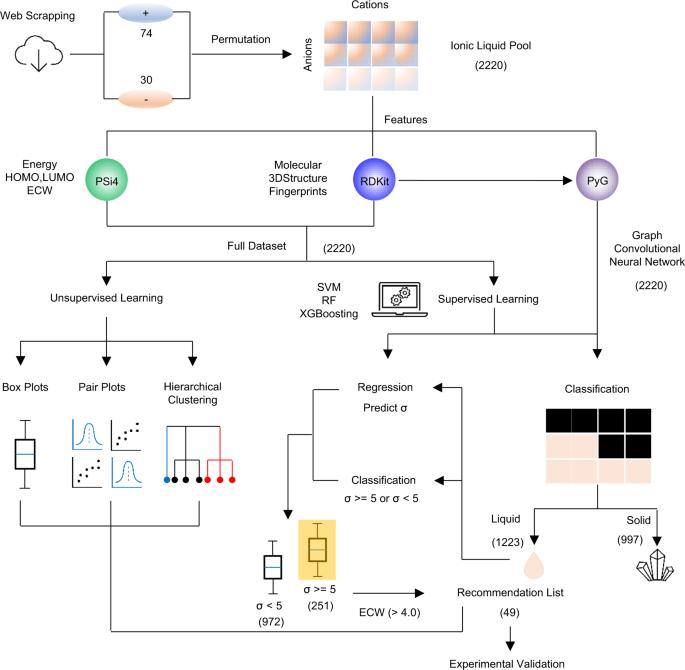
Integrate AI/ML into material science